orbital diagram for calcium|valence electron configuration for calcium : Tagatay After the electron configuration, the last shell of the calcium atom has two electrons. In this case, the valence electrons of calciumare 2. The elements that have . Tingnan ang higit pa "Just to clarify it, wala akong scandal.” Ito ang sinabi ng sexy actress na si Ivana Alawi, 23, hinggil sa balitang may video scandal daw siyang nag-viral kamakailan.. Sabi pa niya, “Kahit mukha akong ganito, never kong gagawin yun.” Naudyok daw si Ivana na sagutin ang isyu dahil pati ang ina niyang si Fatima Merbella ay nakakatanggap ng .
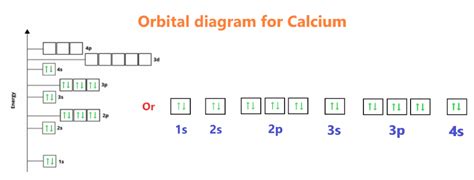
orbital diagram for calcium,Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of calcium is 1s2 2s2 2p6 3s2 3p6 4s2. This electron configuration shows that the last shell of the calcium atom has two electrons. When calcium atoms are excited, . Tingnan ang higit paThe total number of electrons in calciumis twenty. These electrons are arranged according to specific rules in different orbitals. . Tingnan ang higit paorbital diagram for calciumScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons . Tingnan ang higit pa
valence electron configuration for calciumAfter the electron configuration, the last shell of the calcium atom has two electrons. In this case, the valence electrons of calciumare 2. The elements that have . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa
66. Share. 7.8K views 2 years ago. To write the orbital diagram for the Calcium atom (Ca) first we need to write the electron configuration for just Ca. To do . The calcium orbital diagram is a graphical representation of the electron configuration of the calcium atom. This diagram shows how the electrons in the .In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Calcium go in the .
The symbol of the element calcium is Ca, and the atomic number of this element is 20. The atomic weight of the Calcium is 40. The atomic number is significant .
Steps. Here’s how you can draw the orbital diagram of calcium step by step. #1 Find electrons of calcium. #2 Write electron configuration of calcium. #3 Draw . Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. The electron configuration of a Ca ion is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. Calcium .To obtain the orbital diagram, the same procedure is performed, so the orbital diagram of calcium through Kernel will be represented as follows: Calcium isotopes. This chemical .

What is an Orbital Diagram? An orbital diagram is a visual representation of the distribution of electrons in the orbitals of an atom or molecule. It provides information .Introduction. Before assigning the electrons of an atom into orbitals, one must become familiar with the basic concepts of electron configurations. Every element on the Periodic Table consists of atoms, which are .For example, the 5s orbital is of lower energy than the 4d orbital (see Figure 3 for a complete pattern of orbital levels). Ca electron occupancy The orbital notation of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2 Or .To obtain the orbital diagram, the same procedure is performed, so the orbital diagram of calcium through Kernel will be represented as follows: Calcium isotopes. This chemical element has a total of 6 stable isotopes, among which the most abundant is 40Ca, which is considered the same as 40Ar, due to the decay of 40K. .
Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. We start with a single hydrogen atom (atomic number 1), which consists of one proton and one electron. . This gives calcium an outer-shell electron configuration corresponding to that of .The orbital diagram of calcium can be represented as follows: The first shell, or the 1s orbital, can hold a maximum of 2 electrons. In the case of calcium, this shell is fully occupied with 2 electrons. The second shell, or the 2s and 2p orbitals, can hold a maximum of 8 electrons. Calcium has a total of 8 electrons in the 2s and 2p orbitals. The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 5.1.3 or 5.1.4 ). Thus, the electron configuration and orbital diagram of lithium are:For orbital diagrams, this means two arrows go in each box (representing two electrons in each orbital) and the arrows must point in opposite directions (representing paired spins). . calcium is a group 2 element whose neutral atoms have 20 electrons and a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. When a Ca atom . Electron configuration of Calcium (Ca) [Ar] 4s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2: 2, 8, 8, 2: 21: Electron configuration of Scandium (Sc) [Ar] 3d 1 4s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 4s 2: 2, 8, 9, 2: 22: . Orbital Diagram of All Elements (Diagrams given Inside) Subscribe to our newsletter. Subscription Form. Subscribe
Electronic Structure of Atoms. Orbital Diagrams. In orbitals diagrams, the orbitals are shown as boxes, and the electrons in them as arrows pointing up or down. Each box represents one orbital, and each arrow indicates one electron. This is a way of showing the electron configuration of the atom. For example, the orbital diagram of Li can be .The calcium ion (Ca 2+), however, has two electrons less. Hence, the electron configuration for Ca 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6. Since we need to take away two electrons, we first remove electrons from the outermost shell (n=4). In this case, all the 4p subshells are empty; hence, we start by removing from the s orbital, which is the 4s .
Is this the correct atomic orbital diagram for a calcium (2+) ion? Please indicate "true" or "false" accordingly on your Google quiz form.
Such overlaps continue to occur frequently as we move up the chart. Figure 8.3.1 8.3. 1: Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale). Electrons .
An orbital diagram is a representation of the arrangement of electrons in the orbitals of an atom or ion. In the case of calcium (Ca), which has an atomic number of 20, the orbital diagram shows how the 20 electrons are distributed in different energy levels and sublevels. Calcium has a configuration of 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2, which . The figure below shows how a set of three p p orbitals is filled with one, two, three, and four electrons. Figure 5.17.2 5.17. 2: The 2p 2 p sublevel, for the elements boron (Z = 5) ( Z = 5), carbon (Z = 6) ( Z = 6), nitrogen (Z = 7) ( Z = 7), and oxygen (Z = 8) ( Z = 8). According to Hund's rule, as electrons are added to a set of orbitals of .
So if you're thinking about the subshell, the s subshell could fit two electrons, the p subshell can fit six electrons, the d subshell can fit 10 electrons, and the f subshell can fit 14 .Steps for Constructing an Orbital Diagram Atomic Orbital Diagrams. Beginning with your selected element, determine the atomic number. Once the atomic number has been identified, write the electron configuration. As an example, we will use Argon, whose atomic number is 18 and electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6.
To write the orbital diagram of strontium, you have to write the orbital notation of strontium. Which has been discussed in detail above. Strontium (Sr) orbital diagram. 1s is the closest and lowest energy orbital to the nucleus. .

The Bohr model of Calcium (Ca) is drawn with four electron shells, the first shell contains 2 electrons, the second shell contains 8 electrons, the third shell contains 8 electrons and the fourth shell contains 2 electrons. Calcium is neutral and its atomic number is 20, hence, the number of protons and electrons available for its Bohr diagram .
orbital diagram for calcium valence electron configuration for calciumA molecule must have as many molecular orbitals as there are atomic orbitals. Figure 9.7.1 9.7. 1: Molecular Orbitals for the H 2 Molecule. (a) This diagram shows the formation of a bonding σ 1s molecular orbital for H 2 as the sum of the wave functions (Ψ) of two H 1 s atomic orbitals.
orbital diagram for calcium|valence electron configuration for calcium
PH0 · valence electron configuration for calcium
PH1 · orbital filling diagram of boron
PH2 · orbital filling diagram for nitrogen
PH3 · orbital filling diagram for calcium
PH4 · orbital diagrams chemistry worksheet
PH5 · orbital diagram worksheet with answers
PH6 · orbital diagram calculator
PH7 · Iba pa
PH8 · 3p orbital notation for calcium